Deruster
Objects made of ferrous metals will develop rust over time, especially if they are not in constant use. Once rust has formed, there is no protection against further decomposition.
DATA & FACTS
Sector:
Chemistry
Renewable resource:
Microorganisms
Participating companies:
ASA Spezialenzyme
Bioeconomy factor:
Microorganisms produce "iron-eating" siderophores and replace polluting acids
Status:
on the market

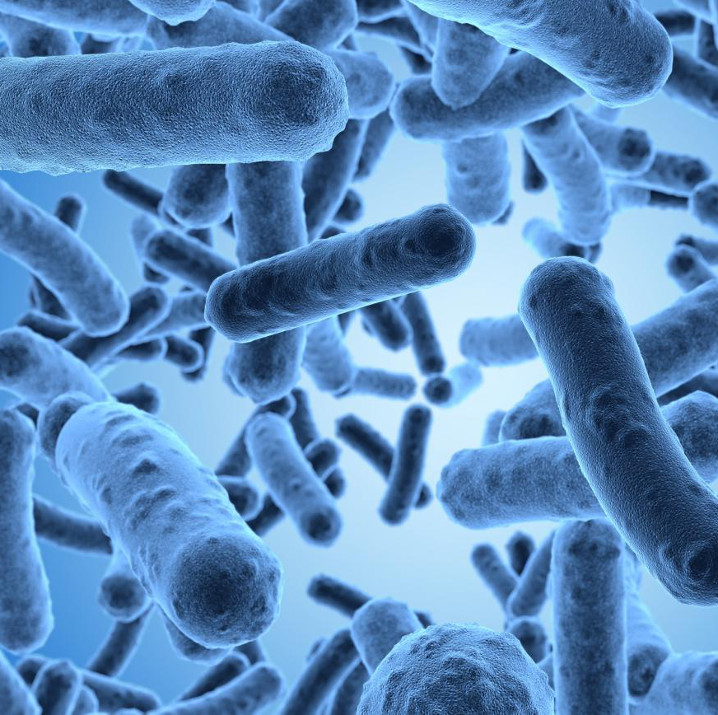
Counteracting rust
The brown coating is formed when iron oxidizes. Rust is not only unattractive, it also causes damage because it continues to decompose the metal. Conventional rust solvents contain acids as an effective component, usually phosphoric or hydrochloric acid. These reverse the process and stop the formation of rust. Acids, however, are dangerous to handle and pollute the environment.
"Iron carriers" as a sustainable alternative
This led scientists at the Mannheim University of Applied Sciences to think about biological alternatives back in the 1990s. They came across the so-called siderophores (large iron carriers). These are iron-binding substances that are formed and excreted by microorganisms, among others. Attempts to use the "iron carriers" for metal derusting were successful. In a joint research project with the company ASA Spezialenzyme, it was possible to develop a bacterial strain that produces siderophores in high yields.
Siderophores are completely biodegradable. In addition, they selectively bind the iron ions of the rust layer and do not attack the underlying iron.
Ready for the market
In the following years, ASA Spezialenzyme was able to develop a gel which is today distributed worldwide by Adolf Würth GmbH & Co. KG is distributed worldwide. ASA Spezialenzyme also offers an immersion bath with siderophores.
Weitere Produkte