Sustainable ethylene production with bacteria
Researchers from Marburg and Kaiserslautern have elucidated the structure of a bacterial enzyme that is capable of producing the basic chemical ethylene without releasing CO2.
Ethylene is one of the most important raw materials in the chemical industry and is used, among other things, in the production of numerous plastics such as polyethylene (PE). However, the production of platform chemicals based on fossil raw materials generates large amounts of greenhouse gases. Researchers at the Max Planck Institute for Terrestrial Microbiology in Marburg and the Technical University of Kaiserslautern have now discovered a bacterial enzyme that could be the key to sustainable ethylene production without CO2 emissions.
Structure of the bacterial enzyme elucidated
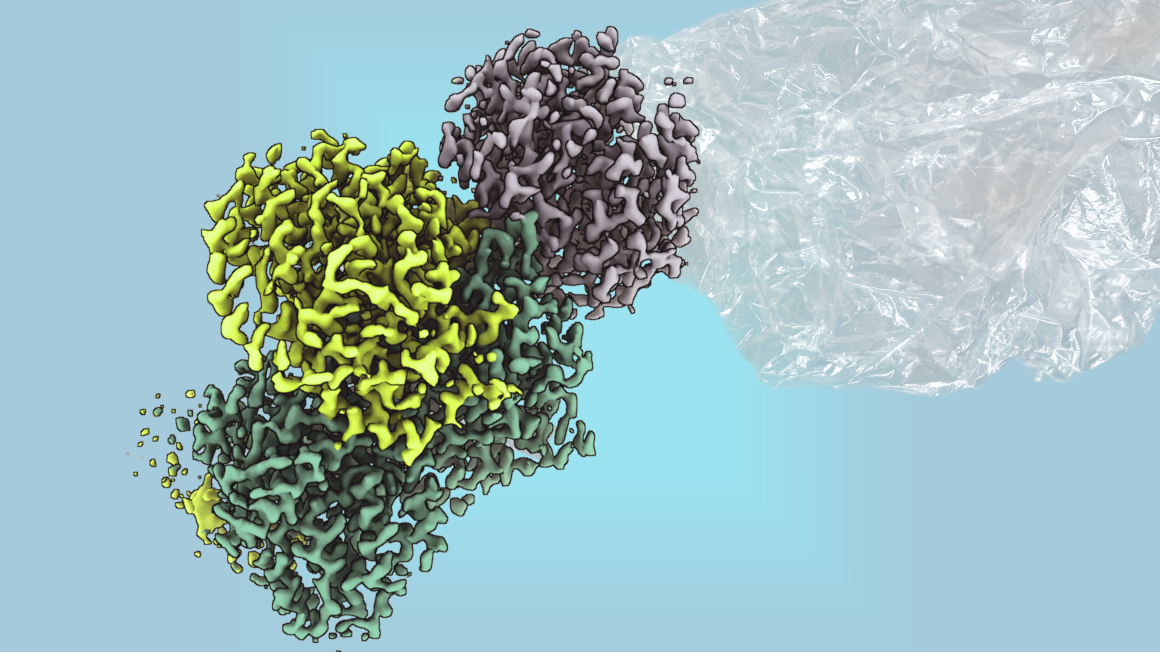
Several years ago, researchers discovered the enzyme methylthioalkane reductase in the bacterium Rhodospirillum rubrum, which enabled the microbe to produce ethylene under oxygen-free conditions without generating CO2. However, it was previously unclear how the catalytic mechanism of oxygen-free production in the so-called metalloenzymes outside cells works. Under the leadership of Marburg-based Max Planck researcher Johannes Rebelein, scientists have now succeeded in purifying the enzyme methylthioalkane reductase from the bacterium outside the cell and elucidating its structure.
Ethylene production without CO2 release
Under oxygen-free conditions, the enzyme produces ethylene without releasing CO2. As the researchers report in the journal Nature, it surprisingly uses large, complex iron-sulphur clusters for this purpose, which until now were only known to exist in nitrogenases. ‘The enzyme is the first non-nitrogenase enzyme known to contain these metal clusters,’ explains Ana Lago-Maciel, doctoral student and first author of the study. Nitrogenases are the only known enzymes that make nitrogen from the air usable for biological processes.
Versatile enzyme with great potential for plastic production
According to the study, the bacterial enzyme can form other hydrocarbons such as ethane or methane in addition to ethylene. This could make it very important for biotechnological processes. ‘In fact, the enzyme has remarkable versatility,’ explains project leader Rebelein. The team sees this as a possible basis for new, CO2-free processes for the production of plastics. ‘Our work provides the basis for taming these enzymes biotechnologically and adapting their product range to our needs,’ says Rebelein.
bb