Understanding the enzymatic binding of carbon dioxide
Researchers have elucidated the structure of the enzyme complex iron nitrogenase in order to better utilize its function.
Nitrogen is an important nutrient for all living things. Our atmosphere is full of it, but the only living organisms that can bind and use this nitrogen directly are some microorganisms. They use certain enzymes called nitrogenases to do this. But researchers are also interested in these enzymes for a second reason: nitrogenases can bind carbon dioxide and carbon monoxide and form methane or ethylene from them. This turns problematic waste materials into valuable chemical resources.
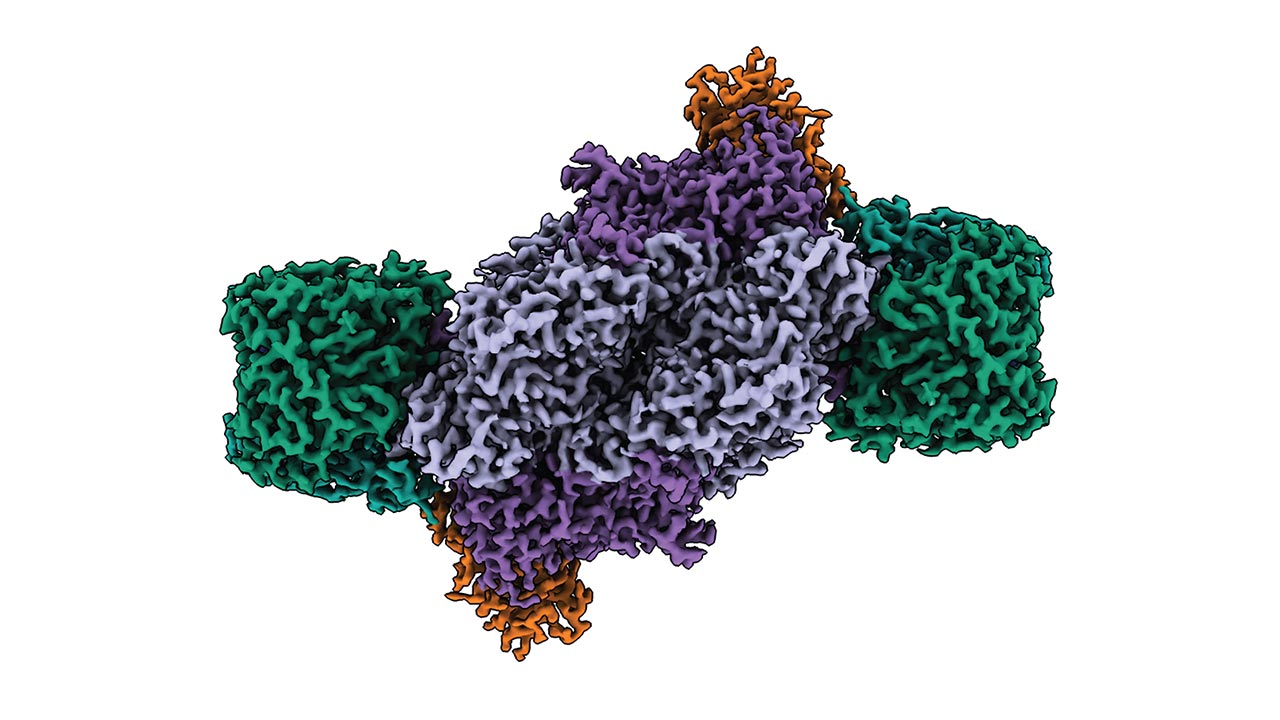
Structure of iron nitrogenase from purple bacteria
How the enzyme works in detail was previously unknown. Understanding this, however, would be important in order to further optimize it and make better technical use of it. A team led by Johannes Rebelein from the Max Planck Institute for Terrestrial Microbiology in Marburg has now succeeded in deciphering the structure of iron nitrogenase. Iron nitrogenase is particularly good at utilizing carbon dioxide.
For their experiment, the researchers used an enzyme from the purple bacterium Rhodobacter capsulatus. The scientists analyzed the purified enzyme using cryogenic electron microscopy at the Central Electron Microscopy Facility at the Max Planck Institute of Biophysics in Frankfurt am Main. The analysis focused on possible peculiarities in the active center of the enzyme complex, which catalyzes the actual reaction.
Cofactor is the only metal that contains iron
There it was found that the cofactor in the active center only contains iron, but no other metal - a clear difference to other nitrogenase types. Otherwise, however, the active site was surprisingly inconspicuous: "We had expected that the main difference between Fe nitrogenase and other nitrogenase forms such as molybdenum nitrogenase would lie in the architecture of the cofactor in the active site and its immediate surroundings," reports Frederik Schmidt, first author of the study published in the journal "Nature Structural & Molecular Biology". "To our surprise, however, we found that the active sites of the three nitrogenase isoforms are very similar, despite their differences in catalytic properties."
Special symmetry and important subunit
However, the structural analysis revealed special features elsewhere: Nitrogenase is composed of two symmetrical halves. The form of symmetry in iron nitrogenase differs from that of molybdenum nitrogenase, though. "The altered symmetry that we observed in Fe nitrogenase could explain its particular reactivity," explains Luca Schulz, co-first author of the study.
The already known G subunit of the enzyme complex could provide a further explanation. It is a special feature of iron nitrogenase. Its structure suggests three possible functions: it could coordinate electron transfer, channel the substrate and also stabilize the cofactor in the active center.
Further clarification of exactly how iron nitrogenase works should contribute to the future development of new biocatalysts that could sustainably utilize nitrogen or use CO2 to produce valuable substances.
bl