Deep-sea enzyme masters PET degradation
The enzyme PET46, which originates from archaea, decomposes medium- and long-chain plastic molecules, as a research team from Germany has discovered.

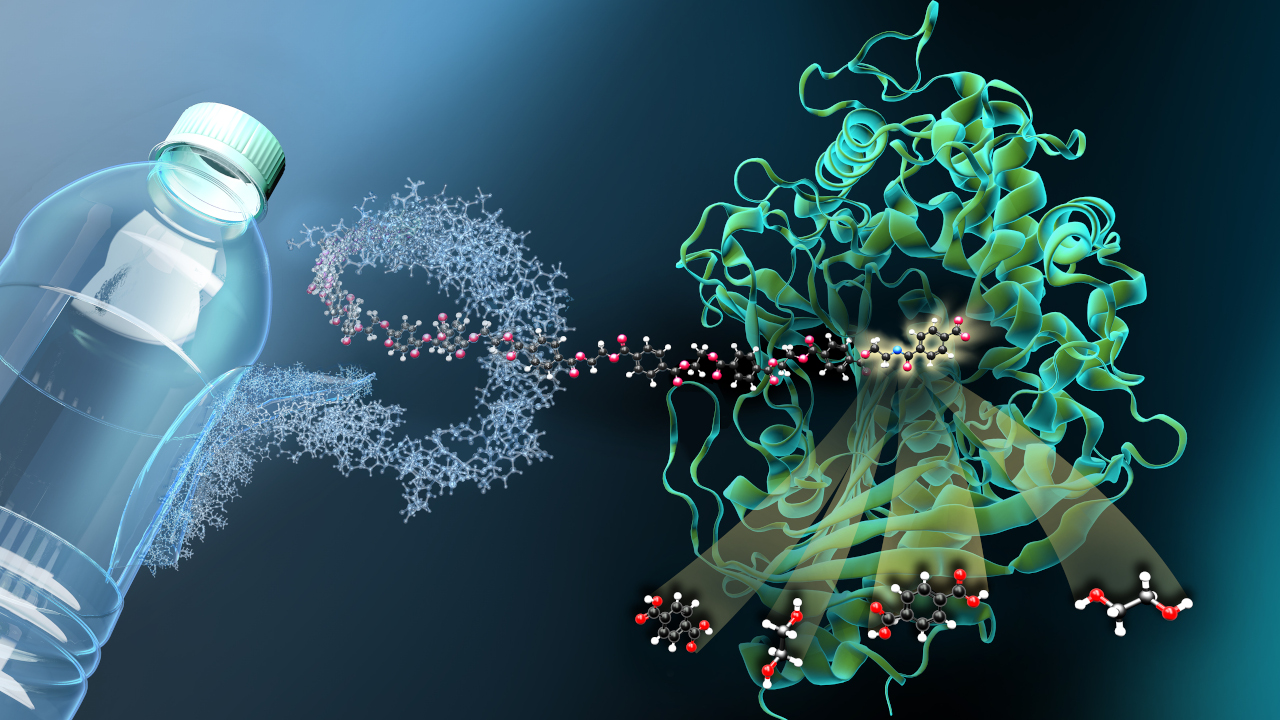
Deep in the sea, there is apparently another biological solution to the global plastic waste problem: researchers from the universities of Kiel, Hamburg and Düsseldorf have discovered an enzyme in a microorganism from the deep sea that breaks down the plastic polyethylene terephthalate, or PET for short. PET is the main component of many plastic bottles.
Importance of deep-sea archaea for marine PET degradation
"In our study, we discovered a new genetic resource from deep-sea organisms from the realm of archaea," reports Ruth Schmitz-Streit, head of the Molecular Biology of Microorganisms working group at Kiel University. PET-degrading enzymes are no longer a rarity: research now knows of more than 80 different enzymes with this ability. However, most of them originate from bacteria and fungi and not, like the one now discovered, from an archaea.
"Our data contribute to expanding knowledge about the ecological role of deep-sea archaea and the possible decomposition of PET waste in the sea," is how the microbiologist classifies the significance of the discovery, which the team reports on in the journal "Communications Chemistry".
Unusual structure of the reactive centre
The researchers did not identify the enzyme by cultivating the deep-sea microbe in the laboratory - this attempt often fails because the appropriate conditions cannot be created in the laboratory. Instead, the team analysed the entire DNA of all organisms in a water sample from the deep sea. In doing so, the experts looked for similarities to the genes of the known PET-degrading enzymes. The researchers then implanted the matching gene into the model bacterium Escherichia coli to produce the enzyme.
The team then examined the enzyme, known as PET46, in more detail. It turned out that it is structurally very different from the PET-degrading enzymes known so far. In particular, the reactive centre has a kind of lid through which it can bind the substrate particularly well. The enzyme is thus able to degrade both short-chain PET oligomers and long-chain PET polymers.
Significant for composting and natural degradation of plastic waste
The now discovered enzyme is thus of threefold importance: It could be used biotechnologically to recycle PET plastics or to compost them industrially: Unlike many PET-degrading enzymes, PET46 works efficiently at temperatures of 70 degrees Celsius. In addition, the enzyme in the sea is likely to ensure that PET waste is biodegraded. Last but not least, PET resembles the wood component lignin just as the PET46 enzyme resembles the lignin-degrading enzyme ferulic acid esterase. On land, PET46 could thus be involved in the composting of wood in the forest soil.
The research project was part of the PASTISEA project, which was funded by the Federal Ministry of Education and Research (BMBF) as part of the BioProMare funding measure.
bl