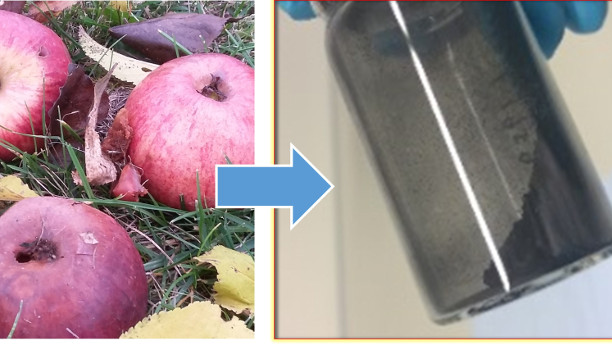
Batteries made from apple biowaste
Lithium-ion batteries are the energy source for many electronic devices. But the alkaline material is expensive and its extraction is damaging to the environment. A much more environment friendly and cost effective alternative, however, are sodium-ion batteries.
Sodium is found naturally and in abundance in nature as table salt. In the search for improved materials for this new generation of batteries, researchers from Ulm Helmholtz Institute of the Karlsruhe Institute of Technology have struck lucky – on the compost heap. They have developed carbon-based active material for the negative electrodes from apple bio waste. For the positive electrodes, a material made out of layered oxides is used to create the positive cathodes. Both materials were convincing in the test showing “excellent electrochemical properties”, as reported by the team in the industry journal “ChemElectroChem” (2015, Online publication) and “Advanced Energy Materials” (2015, online publication).
Whether for mobile phones, laptops or tables, lithium-ion batteries are energy sources for many electronic devices. But the extraction of lithium is laborious and expensive. For a long time there was no alternative to the powerful mini motors. But now the alkaline material has competition. Sodium-ion batteries are not clearly more powerful than sodium-ion batteries will soon be able to outrun the popular storage giants. The reason: sodium-ion batteries are not only considerably more powerful than systems such as nickel metal hydride, lead acid batteries or lithium-ion technology, in comparison to lithium, there is a unlimited supply of sodium in nature. Because it is also more biodegradable it’s therefore cheaper. .
Carbon from apple biowaste
A group of scientists headed by Stefano Passerini and Daniel Buchholz from the Helmholtz-Institute Ulm of the Karlsruhe Institute for Technology (KIT) have now developed two new active materials for this promising generation of batteries. For the negative electrode, they created a carbon-based material, which is obtained from apple residues, for example, that are produced during the pressing of juice. Under exclusion of air, the carbon develops a consistency that is especially suitable for the battery electrodes. According to the study, the new material was convincing in over 1,000 charge and discharge cycles both with a high cycle stability and high capacity.
Cobalt replaced by sodium oxide
For the positive electrode, the team developed a material, which is comprised of different layers of sodium oxide. The advantage here: the active material does not require the expensive and environmentally unfriendly element cobalt, which is an important component of the commercial lithium-ion batteries. This new active material in which the actual electrochemical energy storage takes place, was also convincing in laboratory tests and could achieve the same performance in over a hundred cycles in terms of efficiency, cycle stability, capacity and voltage, such as the cobalt-loaded lithium-ion batteries. With the development of these two sustainable materials, the scientists hope it will be a crucial step on the way to the development of cheaper and more environmentally friendly sodium-ion batteries.
© bioökonomie.de/bb