The world population is growing, and with it, the demand for meat products. While meat consumption is declining in Germany, demand is rising in developing and emerging countries. However, conventional meat production – particularly livestock farming – has long been criticized for its negative impacts on the environment and climate. Companies and research institutions are therefore working intensively on so-called lab-grown meat, which can match the animal original both in taste and structure. Start-ups like Innocent Meat from Rostock are considered innovation drivers in this field.
The company, led by the founding duo Laura Gertenbach and Patrick Inomoto, focuses on developing innovative production systems for cell-based foods. “We develop automated processes and facilities for the meat industry while also providing the necessary consumables – from cells and cell culture media to scaffolds, which are essential for the proliferation of cultivated cells,” explains Inomoto, Technical Director at Innocent Meat and head of the ZUKUNFT project.
Developing edible, scalable, and cost-efficient scaffolds
The project focused on developing edible, scalable, and cost-efficient scaffolds that can be directly integrated into cultivated meat products. The collaborative project was funded by the Federal Ministry of Education and Research from October 2022 to December 2024 with nearly €271,000 under the funding program “KMU-innovativ: Bioökonomie”.
To produce cell-based meat, stem cells are obtained from animal muscle cells using biotechnological methods and cultivated in a nutrient medium – without killing an animal. “One of the biggest challenges currently is the scalability of this technology. Industrial-scale production has so far reached technical limits,” explains Inomoto.
Forming complex tissue structures with scaffolds
With the development of “scaffolds,” Innocent Meat and researchers from the Chair of Microfluidics at the University of Rostock pursued a new approach. Scaffolds are three-dimensional structures that support cell growth and enable the formation of complex tissue structures such as muscle and fat. “These scaffolds are a prerequisite for the large-scale proliferation of primary stem cells for cultivated meat production,” says Inomoto.
For the production of microcarriers, the team used adherent adult stem cells, specifically myosatellite cells. These tissue stem cells, found in both humans and animals, can proliferate and then differentiate into muscle or fat tissue. “You can think of these cells as dormant reserves between muscle fibers. Biological signals – such as an injury or intense strength training – activate them, transforming them into new muscle tissue while a small portion replenishes the reserve,” explains the project leader. The team replicated this natural process in the project.
The process began with isolating cells from pig muscle tissue, provided by a slaughterhouse. The pig cells were then cultivated in a bioreactor. To proliferate optimally, the cells required not only nutrients but also sufficient surface area for attachment and growth. This is where the scaffolds developed in the project were intended to be used.
Macroporous scaffolds for optimal cell growth
The core idea was to develop macroporous microcarriers – structures with pore-like cavities, similar to the holes in cheese. These pores needed to be large enough for precursor cells to enter and attach to internal surfaces. They also had to be interconnected like a tunnel system, giving cells enough space to grow and proliferate and ultimately generate maximum biomass – that is, cultivated cells.
Separating the resulting biomass from the scaffold without significant effort would, according to Inomoto, involve high costs and complex processes. “That’s why we decided to use an edible material as a scaffold, which can remain in the final product.” Creating a scaffold that was both porous and edible had not been achieved before – a challenge the research team set out to solve.
Edible scaffolds from alginate
At the University of Rostock, various materials were tested to develop such a scaffold. The team ultimately chose alginate, a natural substance derived from brown algae, which is already used as a gelling and thickening agent and for sausage casings. “Alginate is ideal because it mimics the texture of meat very well and is already approved as a food ingredient,” explains Inomoto.
Two different technologies were tested to produce alginate scaffolds. While electrospinning was used at the Chair of Microfluidics, Innocent Meat focused on cryogelation. “We applied both methods, produced scaffolds, and tested them in cell culture. Ultimately, we chose cryogelation because it offers higher scalability and better functionality.”
In cryogelation, droplets of an alginate solution are dropped into a supercooled solvent, where they freeze and form small spherical structures with embedded ice crystals. “These structures are then crosslinked and solidified – similar to how gelatin works in the kitchen,” explains Inomoto. “When thawed later, the ice crystals melt, leaving a porous network.”
Tissue content of alginate spheres at 90%
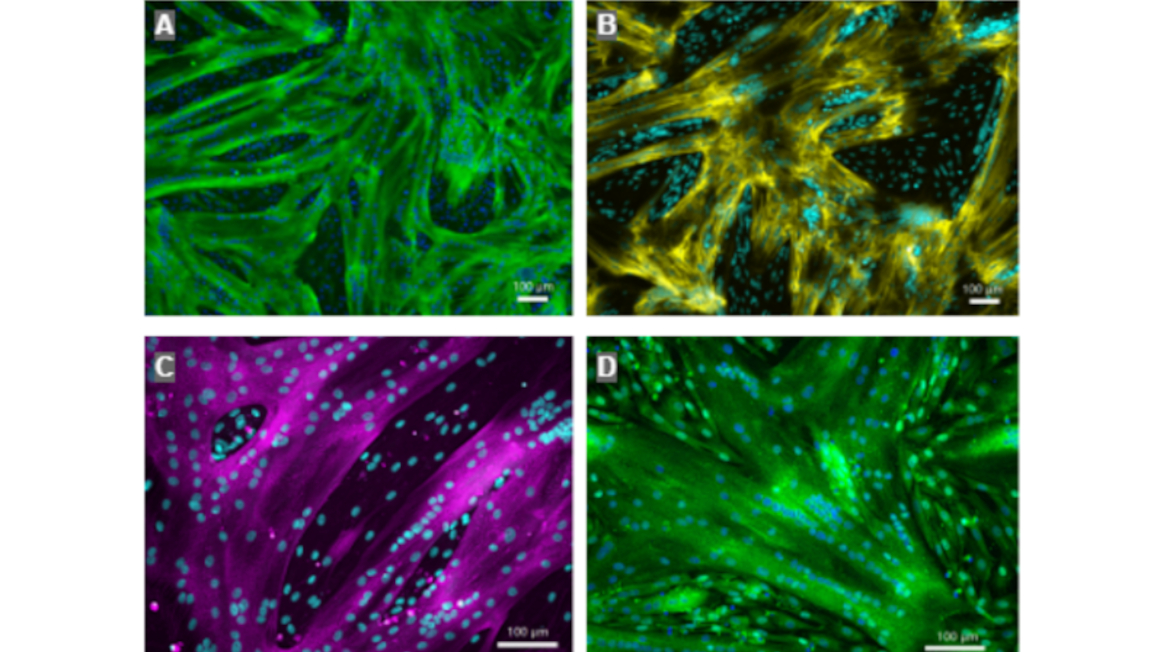
The resulting porous alginate scaffold provided precursor cells with enough space to proliferate and generate sufficient biomass. The key advantage: “These scaffolds can be added as needed, allowing us to scale production step by step,” emphasizes Inomoto. After differentiating the stem cell biomass into muscle and fat tissue, the alginate spheres contained 90% tissue and less than 5% alginate. “The resulting tissue is fully functional muscle tissue, biologically indistinguishable from the animal original. The nutrient composition is also nearly identical to conventional meat,” summarizes the project leader.
Project ZUKUNFT - division of tasks
Innocent Meat: Project management, optimization of scaffold surfaces, testing of cell cultures, further development of cryogelation
Universität Rostock, Institut of Microfluidics: Testing of materials for scaffold production
In the laboratory, this process produced 100 grams of cultivated pork. According to Inomoto, this is not a solid piece of meat but a “pasty mass,” which can be used to produce sausages or meatloaf. “The texture of this processed product is comparable to the original.”
Next, the research team plans to further optimize the pilot plant developed in the project for edible scaffold production and scale the technology toward industrial levels. Currently, the plant can produce about 10 liters of scaffolds. “With this, we can fill up to 50 liters of reactor volume and produce several kilograms of meat,” says Inomoto.
Demonstration facility planned in Mecklenburg-Vorpommern
Together with the University of Rostock research team, Innocent Meat has laid the foundation for cellular agriculture. “Next year, we plan to build a demonstration facility in Mecklenburg-Vorpommern, where we can produce tons of cultivated meat. At the same time, we aim to commercialize individual products for cell culture – such as the developed scaffolds. This way, our technology can help other companies in the food and pharmaceutical industries develop and implement scalable, cost-efficient processes,” says Inomoto.
Author: Beatrix Boldt