Enzyme for improved CO2 utilisation discovered
A Max Planck team from Bremen has studied a methane microbe living in oil fields that can elegantly convert CO2 into formic acid.

Not releasing carbon dioxide from industrial processes into the atmosphere, but upgrading it chemically, this approach is intended to make a small contribution to the climate neutrality of the economy. Compared to possible eternal storage facilities for CO2, this is also economically interesting because no disposal costs are incurred and a profit can even be made. Accordingly, intensive research is being conducted on appropriate CO2 utilisation processes. A team from the Max Planck Institute for Marine Microbiology in Bremen has now discovered a particularly promising approach and presented it in the scientific journal "Angewandte Chemie" ("Applied Chemistry").
Archaee aus dem Umfeld von Ölfeldern
In nature, there are some microorganisms that can fix CO2 from the air and use it for their metabolism. Various research groups have identified such organisms in recent years and developed biotechnological processes partly based on them. The Bremen team relies on the archaea Methermicoccus shengliensis, a methane-producing microbe that lives in the water around deep-sea oil wells and feels comfortable at temperatures of 65 degrees Celsius.
First, the researchers identified the enzyme that converts CO2 into formic acid. Formic acid is interesting for the chemical industry because it serves as a starting material for many chemical compounds and can also be used as an energy store. "We knew that such enzymes are sensitive to oxygen. Therefore, to separate it from other proteins, we had to work under an oxygen-free bonnet without ambient air – quite complicated, but we succeeded," Olivier Lemaire reports.
Enzyme mainly converts the outward reaction
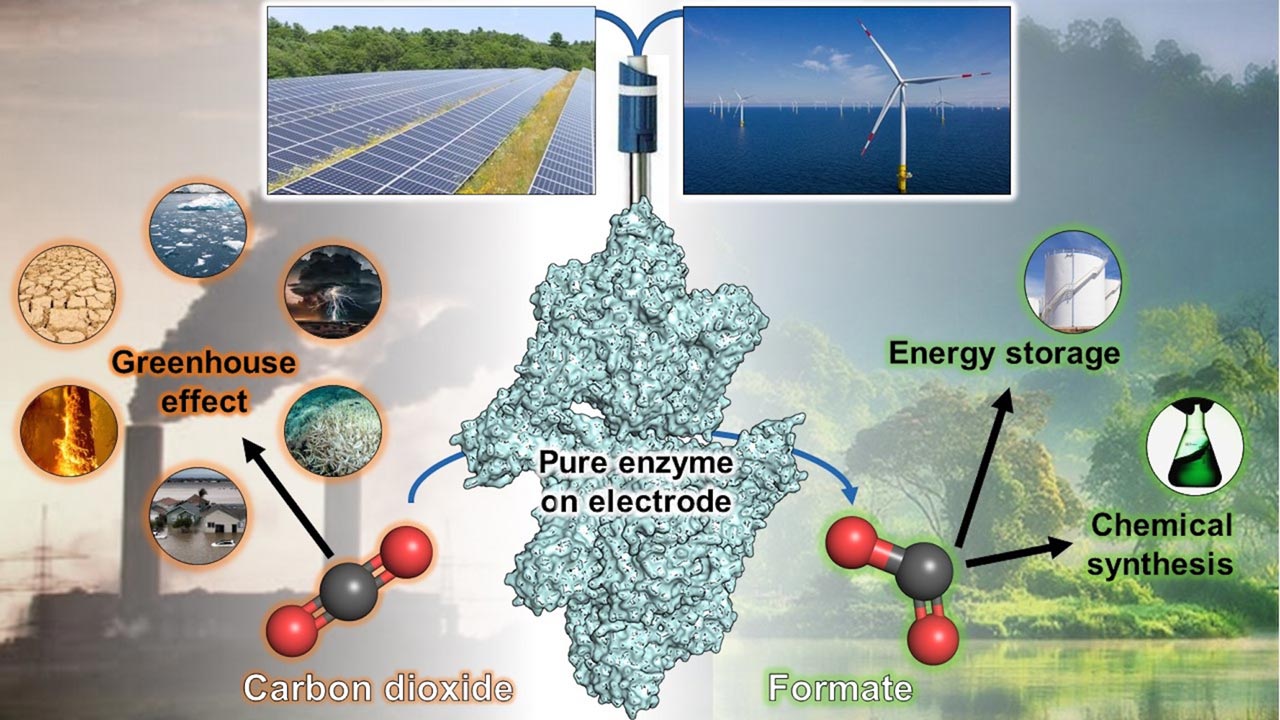
The special feature of the enzyme quickly became apparent: unlike the formic acid dehydrogenases of other microorganisms, there is practically no reverse reaction from formic acid to carbon dioxide. "The fact that the formic acid produced during CO2 fixation cannot be converted back and therefore accumulates is extremely interesting for a possible CO2 capture system, especially if we could mount it on an electrode," Tristan Wagner emphasises.
Bound to an electrode, the enzyme could draw its required energy for the conversion reaction from the current. Compared to other energy sources, this is particularly favourable and leads to efficient reactions. And compared to the chemical synthesis of formic acid on an electrode, the enzyme does not need any toxic catalysts. A team at the University of Geneva has therefore taken up the challenge and developed an electro-biotechnological process with this enzyme.
Reaction without detectable by-products
The researchers succeeded in coupling the enzyme to a graphite electrode. The rate at which the fixed enzyme converted carbon dioxide matched that of other formic acid dehydrogenases. "The strength of this biological system coupled to the electrode is how efficiently it transfers electrons from electricity to CO2 conversion," Lemaire points out. And in this system, too, there was virtually no reverse conversion of formic acid. Instead, it continuously converted CO2 to formic acid without producing any detectable by-products or losing electricity.
"Before us, no one had ever tried to use an enzyme from such a methanogenic microbe for an electrode-based gas conversion," says Wagner. Nevertheless, there are still many process-related problems to be solved before large-scale use can be considered.
bl