Enzyme filmed in action
Molecular cinema: German and Canadian structural biologists have observed an enzyme at work and produced a time-lapse film.
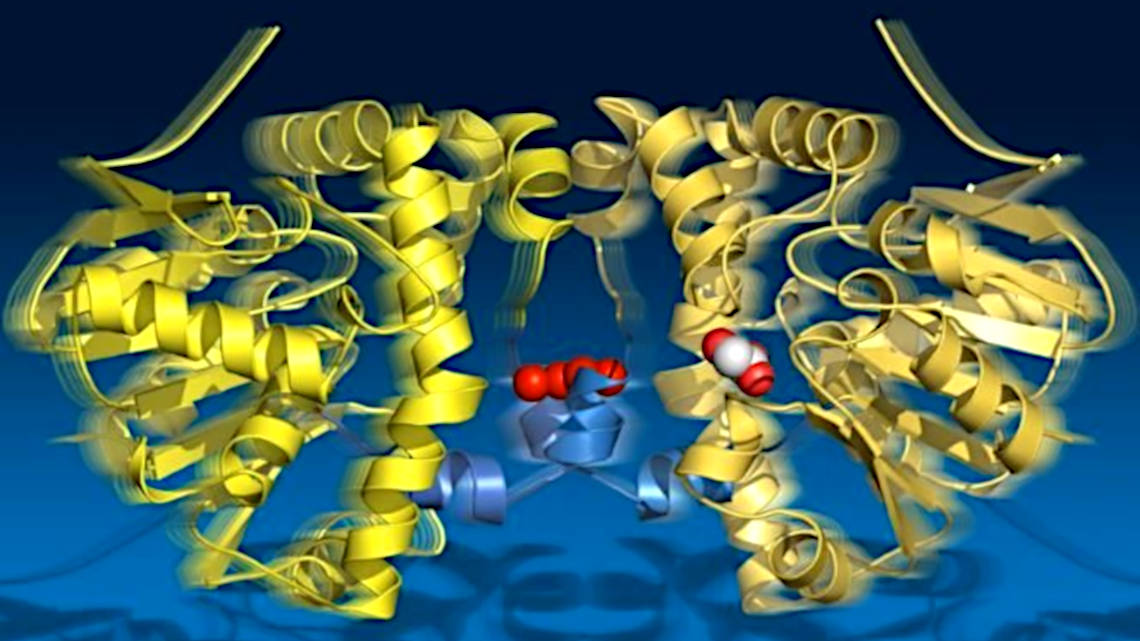
The bond between a fluorine atom and a carbon atom is the strongest single-bond in organic chemistry. Splitting it would be a reaction that occurs automatically over a period of years, but some enzymes shorten the reaction to a few seconds. Scientists from the Max Planck Institute for the Structure and Dynamics of Matter (MPSD) in Hamburg, the University of Potsdam and the University of Toronto in Canada have now documented in a highly detailed time-lapse film what exactly happens during this reaction in the enzyme fluoroacetate dehalogenase. In the science journal "Science", they report on this and on a hitherto unknown principle that they discovered in the process.
18 Snapshots of the catalysis cycle
Using X-ray crystallography, the researchers took 18 snapshots over 30 seconds of catalysis and recorded four cycles with all intermediate states of the reaction. The images show how the substrate is first bound, an intermediate product is formed, a hydrolytic water molecule is provided, and finally the reaction product leaves the enzyme.
A signal chain of water molecules
The researchers also observed that the enzyme "breathes" during turnover: it expands and contracts aligned with the catalytic sub-steps. The two halves of the enzyme remain in contact via a connecting cord of water molecules. Apparently, they share information about their catalytic state. This finding is of great importance for the reaction, since only one half of the enzyme can be catalytically active at any one time.
Significance for climate protection and environmental remediation
The scientists were particularly interested in the compound between fluorine and carbon because it is highly relevant for the greenhouse effect. This compound also plays an important role in materials such as Teflon and GoreTex, in pharmaceutical products and pesticides. The aim of the study was therefore to understand the turnover of such compounds and, ideally, to actively control it.
According to the scientists, the dynamic movements of the enzyme that have now been observed may also have correspondences in many other enzymatic catalyses.
bl/um