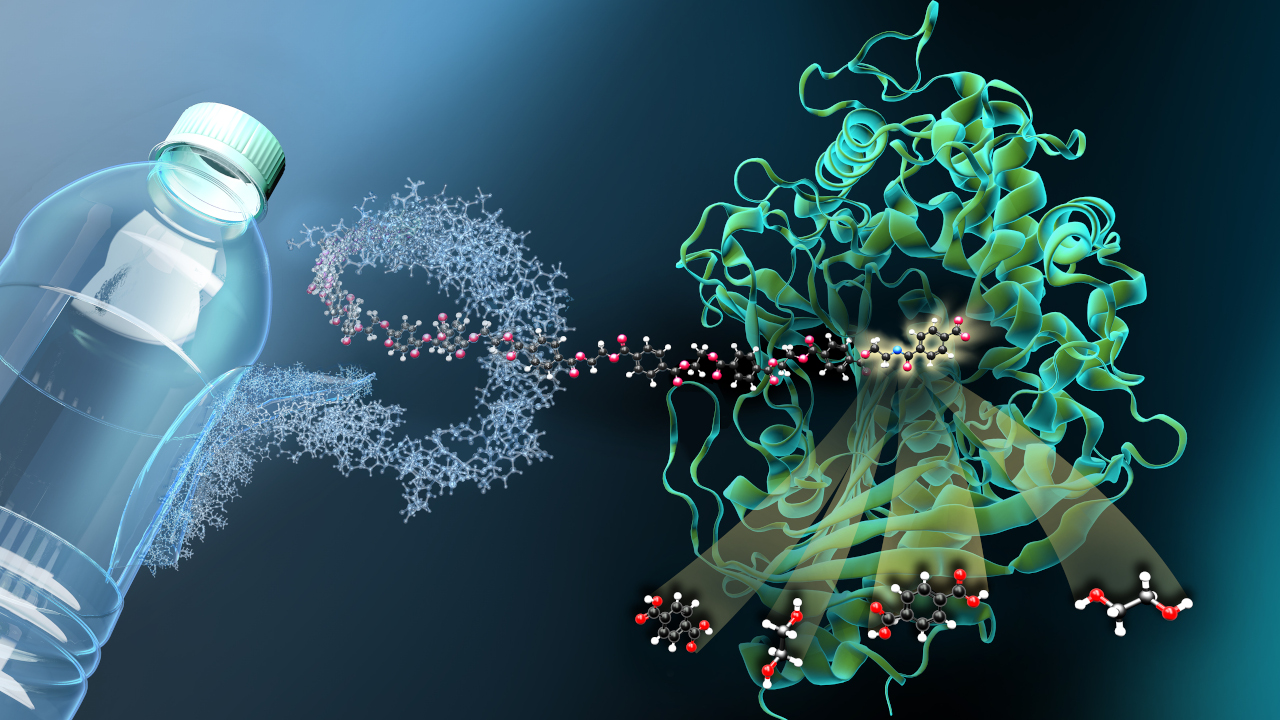
Proteins are a key tool of the bioeconomy. Among other things, biocatalytically active proteins (enzymes) can produce an incredible variety of complex biomolecules with potential applications ranging from basic chemicals and fuels to food additives and pharmaceuticals. Sometimes these processes are attractive because the molecules would be too complex for conventional chemical synthesis. Sometimes they are because of their energy-saving and environmentally friendly process conditions. And sometimes it is due to the bio-based raw and residual materials that the enzymes use, whereas chemical processes almost always require petroleum.
Product purification as a challenge
As versatile and advantageous as the biological synthesis of valuable substances is, it requires not only identifying and genetically optimizing appropriate enzymes, but also efficient production and purification of the target protein, because the single-celled organisms used for protein production also produce a lot of other compounds. "There are thousands of proteins in a microorganism," says Martin Dippe of the Leibniz Institute of Plant Biochemistry in Halle (Saale). "From these, we have to separate the target product." Because this task is so commonplace in biotechnology, numerous processes for it have been established on the market. "They all have advantages and disadvantages," explains Dippe. "We are confronted with these every day when we purify our proteins." That was not satisfactory for Dippe and his colleague Pascal Pecher. So the ProMin research project was born, which the German Federal Ministry of Education and Research is funding with about 400,000 euros.
"The most common and cost-effective purification method is based on enabling the protein to bind to a specific surface structure by means of a molecular attachment known as a tag," Pecher explains. In this method, ions that can interact with the tag of the target protein are tightly bound to a support material, the solid-state matrix. The target protein thus binds to the support material, other proteins can be washed off, and finally the target protein is released from the matrix in a chemical reaction without contamination from other proteins. "The problem here is that the ions used are heavy metal ions," Pecher says. A few of them detach from the matrix along with the target protein. "As a result, the solution is then not directly usable, at least for the food and pharmaceutical industries, and must be laboriously and expensively post-purified."
Biological materials instead of heavy metal ions
Other possible binding elements, so-called ligands, which are used as an alternative to heavy metal ions, include antibodies. "However, these methods are usually very complex and highly expensive," explains Pecher, which is why they are unsuitable for large-scale protein purification.
This is where ProMin enters the picture: The research team has developed a new method to purify proteins that does not rely on environmentally harmful heavy metals. "The methodology is the same - affinity chromatography," says Pecher, "but with materials that are much more biologically friendly."
As the researchers mention to Bioökonomie.de, the original motivation was not the environmental problems of industrial processes. Rather, the biotechnologists were bothered by the fact that heavy metals repeatedly interfered with their own research. "We are working on enzyme cascades," Pecher says. " That is, in the direction of artificial metabolic pathways." But the heavy metal ions that entered the solutions during enzyme purification interacted in unfavorable ways with various components of the enzyme reactions. "That's when we thought: There must be another way!"
Qualitatively comparable with existing processes
The team tried and tested around 50 different tags and a range of carrier materials until the experts found what they were looking for. Only a few hits proved to have sufficient binding strength to fish out the target enzymes, while also being resistant enough to the reagents and conditions commonly used in protein purification. The researchers further optimized one of these ligands to make its binding properties suitable for practical and complex applications. Achieving sufficiently high binding strength has long proved to be a stubborn challenge.
"Both our carrier materials and our tags are therefore now completely novel compared to common methods," Pecher emphasizes. He says it is a natural material that, unlike conventional carriers, does not require extensive surface modification. "Strictly speaking, it is a palette of materials in which the strength of the interaction and the procedure to ultimately release the target protein are customizable," the biotechnologist describes, with a sense of pride. "We are pretty sure that our hits are suitable for industrial use," says Pecher, referring to the successful demonstrator trials. Qualitatively, he says, the method is already comparable to existing ones, and the project participants also believe they can compete economically. Ultimately, the method could be used for any protein that is already purified using the affinity method.
Next step: Proof of industrial applicability
Industrial partners are already on board with the project. "We are now at the transition point from laboratory scale to industrial scale, and the material and tags have been optimized to this extent," says Pecher. Without access to industrial-scale equipment, the research team has not been able to conduct any further tests so far. That is now to be done together with the industrial partners during 2022. "By the end of the project, we want to demonstrate industrial applicability," the researcher says confidently. After the end of the project in March 2023, a spin-off is then planned to further market the novel process.
Author: Björn Lohmann