Recorded as the oldest species on the planet, scientists have discovered a bio-silicate in sponges that can be used for new kinds of medical coatings, for example in dentistry or for bone implants. Supported by the Federal Ministry of Education and Research (BMBF), the company NanotecMARIN GmbH has pushed forward the development of biotechnological production processes for the material. The first tests on patients are set to begin.
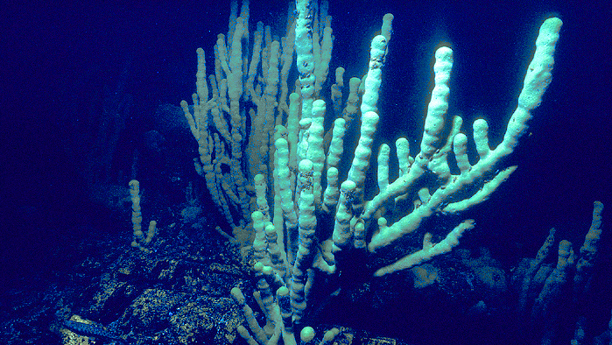
Sponges can survive in a wide range of different conditions
Marine sponges are unique animals that are unassuming, resistant and even outlived the dinosaurs and have been on our planet for 700 million years. What makes sponges unique is that the oldest animals on Earth can be found wherever there is water – from the Amazon to Lake Baikal to the deepest oceans, and in a wide range of different conditions. The 9,000 different species of sponges can be considered true survivors of life on Earth and are a fascinating research object. Werner E.G. Müller is a pioneer in sponge research. The Mainz-based molecular biologist has dedicated 30 years of his life to research into marine sponges, including under the umbrella of the BIOTECmarin competence centre. From 2001 to 2011, and with support from the Federal Ministry of Research, numerous scientists at BIOTECmarin have been investigating the exceptional biological properties as well as chemical structures of the sponges, with the aim of utilising this knowledge for medical applications.
Müller founded the company NanotecMARIN GmbH in 2007. At the time, the researcher was primarily interested in the inorganic skeleton of marine sponges. This is comprised of silicate, which in human beings is also important for bone formation. To date, it has been unclear how this material is produced. From early on in his career, Müller was convinced that there must be a protein that enables the biomineralisation that is witnessed in the sponges, also in abnormal conditions. In the lab, this biomineralisation can only be achieved only under extreme pressure. “By 2009, we knew of no enzyme that could produce such a polymeric inorganic polymer,” explains Müller today. After no small amount of work, the researchers at GmbH NanotecMARIN could verify – and isolate – the enzyme silicatein as the material that sponges utilise to create a crystal-clear bio-silicate out of silicon dioxide molecules. This discovery is likely to be extremely valuable for industry: for production processes that currently require temperatures of over 1800 degrees Celsius, the enzymes in the sponges need only cold water – a process in which they have been experts for millions of years.
Sponge enzyme as an aid for the production of bio-silicates
Aided by the enzyme, it has been possible to develop establish a biotechnological production process for the bio-silicate, meaning that the findings from Werner E.G. Müller and his team have laid the foundations for the industrial production of the biomaterial. “To do this, we first had to locate the silicatein gene,” recalls the University of Mainz professor. With his help, the researchers could produce recombinant silicatein using bacterial bioreactors. It quickly became clear that the extreme stability and the bone-building effect of the bio-silicate makes it especially well suited as a dental surface coating material. These require a special level of insulation because teeth become increasingly sensitive to cold and acid as people age. This tendency can be traced back to progressive age-related demineralisation, which causes capillaries on the tooth surface to increase in size and to thus become more susceptible to decay. By using bio-silicate, it could be possible to lay down a novel sealant in the form of organic protective sheath – or so the idea goes. “We wanted to spur the enzyme into forming a sheathing of silicate around the tooth,” explains Müller. After numerous rounds of testing, the scientists were successful. Under an electron microscope, it could be seen that the capillaries had regenerated under the biocompatible layer of sealing. Muller's company was supported in its work with 180,000 euros as part of the BMBF funding measure ‘SME Innovative’.
Bio dental protection to be tested on patients
The next step is for the bio-silicate to be tested on actual patients. “The approach will be to apply a biocompatible natural silicate surface on to the tooth, which will reduce sensitisation,” explains Müller. At this point it is not clear whether the layer will be applied by brush or spray. In the meantime, there is no shortage of prospects for the new tooth coating, and Müller is optimistic that the biomaterial will be being used in dentistry within three years.
Developing bio-silicate for bone formation
Werner Müller and his team are conducting parallel testing of the bio-silicate on bone implants. This is likewise an area of great potential. Here, the biomaterial would protect the prosthesis before it is recognised by the body as a foreign object, preventing immune rejection. Moreover, in osteoporosis, bio-silicate can balance out the impaired mechanism that regulates the equilibrium between bone formation and bone resorption. In 2010, the Mainz-based expert received a million-strong grant from the European Research Council ERC for the pursuit of this project. “Our primary objective now is to develop three-dimensional and purely biological structures using the bio-silicate, which at some point will be able to assume a bone-forming function in humans. If we succeed, it would be groundbreaking for regenerative medicine,” emphasises Müller. Using this approach, it might be possible to develop materials for implants in the field of tissue engineering.
Author: Beatrix Boldt