Acidic oceans destroy mussel shells
According to researchers in Kiel, the increasing acidification of the oceans cause growing problems for mussels when developing their shells.
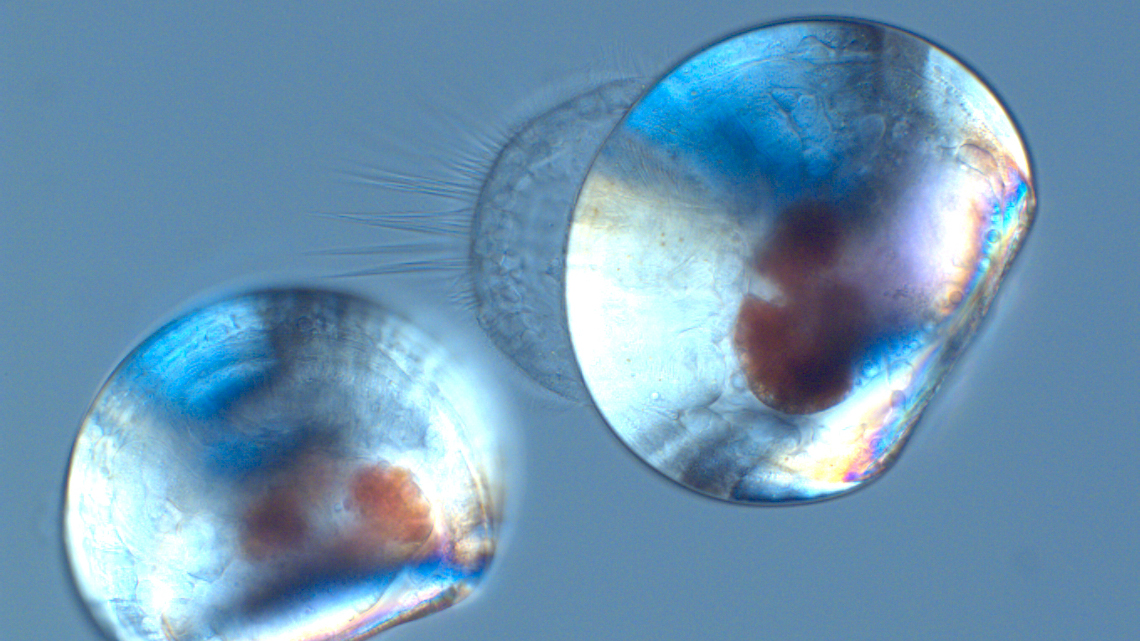
Climate change and global warming causes more than rising sea levels. It also causes an increasing acidification of the oceans. This, however, affects especially those sea creatures with a calcareous shell – including mussels, which live in tidal regions of the coastal zones. Researchers at the Kiel University and GEOMAR Helmholtz Centre for Ocean Research Kiel now report in the journal “Nature Communications” that especially young mussel larvae react sensitively to ocean acidification, which leads to reduced calcification rates and shell dissolution.
Mussel larvae particularly sensitive to acidification
More carbon dioxide in the air also equals more carbon dioxide in the ocean, where it is dissolved in the seawater and results in increasing acidification. This endangers mussels and many other creatures in the oceans, which protect themselves with a calcareous shell from predators. Mussels are especially sensitive to a decline in pH in early life stages. One important reason for this is the enormous calcification rate in the larval stage: between the first and second day of life they form a calcified shell, which corresponds to the weight of the rest of their body. “For the first time, we used two different methods to understand the calcification of one to two-day-old shelled larvae to estimate their sensitivity to climate change”, says Kirti Ramesh, first author of the study.
Mussels can modulate pH levels directly underneath shell
“With the help of fluorescent dyes and specialized microscopy techniques, we were able to track the deposition of calcium carbonate in living larvae and show that calcium carbonate is not formed intracellularly, as previously thought. It is more likely that calcium is acquired directly from the seawater and transported to the shell via specific transport proteins. Then, very close to the shell, the formation of calcium carbonate takes place”, explains Kirti Ramesh. Subsequently, the team studied the abiotic conditions directly under the shell. “For the first time, we have been able to show that the mussel larvae are able to increase the pH and the carbonate concentration below the shell, which then leads to higher rates of calcification ", explains Frank Melzner, Head of the Ecophysiology Working Group at GEOMAR. “However, with increasing acidification, the pH values below the shell also decrease, which leads to reduced calcification rates and, at very high CO2concentrations, shell dissolution and increased mortality occurs”, "Melzner continues.
Calcification rate connected to chemistry of seawater
"With these results, we can establish a direct relationship between the calcification rate of mussels and the carbonate chemistry of seawater," explains Markus Bleich, Head of the Physiological Institute at Kiel University. Previous findings had shown that some mussel populations, especially from the Baltic Sea, are more tolerant to ocean acidification. “We think that the key to increased resistance of mussel shells to dissolution lies in the variation of organic shell constituents”, says Melzner. In the end, these populations that are more resistant could be the winners of climate change.
jmr


