
Whether in smartphones, LED screens or wind turbines: rare earths are indispensable for the high-tech industry. Hardly any electronic device can do without the precious metals. But the raw material is limited and the German economy is dependent on imports, especially from China. Better and more sustainable recycling of rare earths could remedy this situation and would be a first step toward making Germany economically less dependent on imports.
Recycling lanthanides from industrial waters
This is where the PepTight project comes in, which focuses on lanthanides. They belong to the rare earths and comprise a total of 15 metals. Researchers from the Helmholtz Center in Dresden-Rossendorf and the University of Potsdam are currently working on a method to recycle them. "Currently, recycling for these substances works very poorly. The rate for lanthanides is less than 1%," explains project coordinator Björn Drobot from the Institute of Resource Ecology at Helmholtz-Zentrum Dresden-Rossendorf (HZDR-IRE).
Lanthanides are contained in almost all electronic devices - but in low concentrations and not in pure form. A second source of raw materials are industrial waters - such as the flooding waters of the disused uranium mines of Wismut GmbH, which also contain rare earths. Drobot's team wants to extract the lanthanides for high-tech applications from these waters - following nature's example. "Nature's accuracy and selectivity is unsurpassed to this day," the researcher enthuses. "Over the course of evolution, it has developed tools with its building blocks to effectively distinguish even the most similar substances."
Peptides as filters
Accordingly, peptides, i.e., short protein chains, are to be used in the recycling of lanthanides. The aim of the project is to identify chains that bind lanthanides in a highly specific way. Drobot and researchers at LMU Munich have already shown that peptides can distinguish between different rare earths.
The basic building blocks for peptides are 20 different biological amino acids. This means that even for short protein chains there is already an enormous number of possible combinations. It is precisely this diversity that makes protein molecules a universal tool. However, which of these proteins actually selectively bind lanthanides depends on the spatial structure of these chains. To find out, the research team is interdisciplinary. In addition to the IRE, the Helmholtz Institute Freiberg for Resource Technology (HIF) and the Center for Advanced Systems Understanding (CASUS) are also involved in the project, which has been running since 2021. In addition, the working groups Optical Sensors and Spectroscopy (UPOSS), Analytical Chemistry Polymer Materials and Polymer Technologies (UPPP) are participating on the part of the University of Potsdam. The work of the PepTight team is funded until 2024 by the German Federal Ministry of Education and Research within the funding measure "Future Technologies for the Industrial Bioeconomy: Focus on Biohybrid Technologies" with a total of 1.5 million euros.
Identifying the best peptide binders with AI
Complementary strategies are to accelerate the search for the best peptide binders, and the team is also relying on the help of artificial intelligence. The AI experts at CASUS are responsible for this. The researchers at the Dresden HIF, in turn, will use the phage display to select peptides from viruses that bind lanthanides.
Researchers at the University of Potsdam, on the other hand, are using fluorescence spectroscopy to illuminate the lanthanides. "This allows us to distinguish lanthanides and better investigate selectivity," explains the project coordinator. The identification of the 3D structure of the metal-peptide complexes is carried out by the UPAC team. According to Drobot, the 3D structure allows conclusions to be drawn about stability and provides parameters that are in turn needed by the AI. Since not all possible peptides can be fully characterized experimentally, Drobot's team is flanking the work with theoretical chemistry methods.
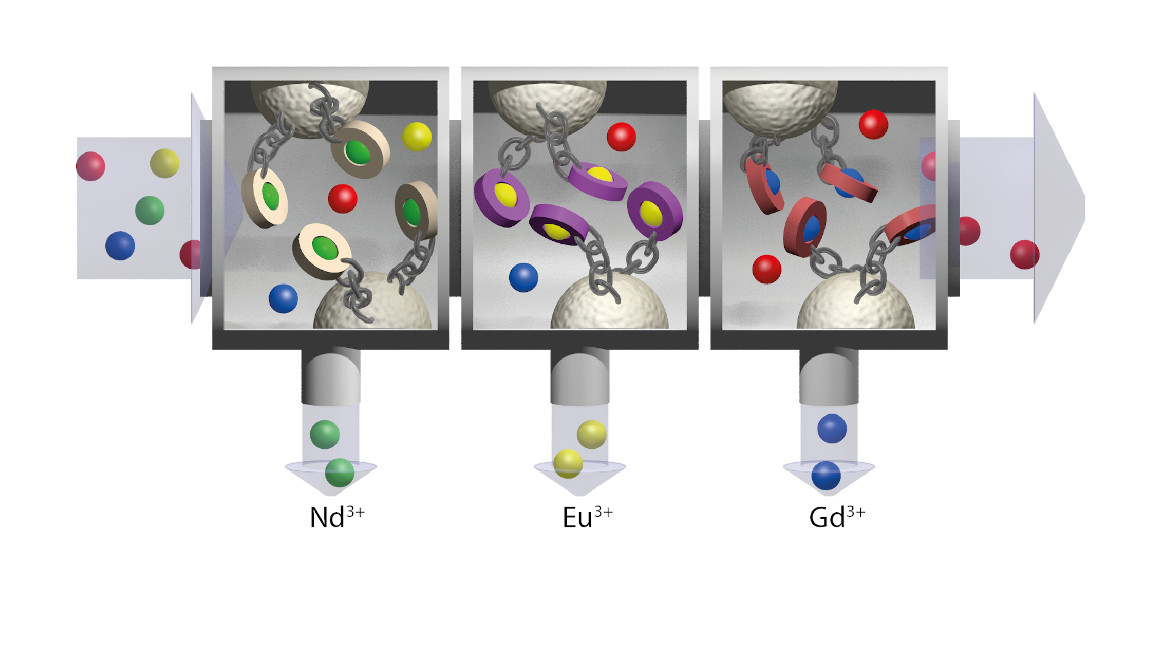
Nature as an example
The research is still in the early stages, but there have already been some small successes. "We can already bind all the lanthanides very well," says Drobot. The challenge, however, is to also separate the lanthanides from each other - because chemically they are all very similar. "There are, of course, chemical processes to separate lanthanides. But they are quite complex and require the use of many chemicals." Recycling along the lines of nature, on the other hand, would be good for people and the environment, avoiding the use of toxic chemicals and instead building a circular economy.
According to Drobot, some promising peptide candidates have already been found. Nevertheless, the search continues. "It is difficult to estimate how far we are from the optimum," Drobot explains. "Not every peptide that binds lanthanides well has the selectivity we need."
Development of modular filters planned
Finding the best possible peptide binders for lanthanides is only the first step: "Even after a suitable peptide has been identified, the work is not yet over," says Drobot. "These still have to be attached to a filter material without affecting the function of the peptides." Therefore, the UPPP working group is on board, contributing its expertise in linking biomolecules to polymers. This should lead to the realization of a modular filter that not only binds lanthanides but also separates them at the same time. "The filter should be modular so that the individual modules can be used in a highly specific way for the individual lanthanides," explains the biophysicist, who holds a doctorate in radiochemistry. Ideally, Drobot hopes, there will be one special filter module for all 14 lanthanides at the end of the project.
Technology transferable to other substances
At the moment, the team is focusing on developing a technology that filters rare earths from industrial water. As early as next year, Drobot hopes to have completed the first filter module. But the Helmholtz researcher is already thinking ahead: "The technology platform can also be transferred to other substances in general. Then, for example, the removal of hormone residues in wastewater treatment would also be an option."
Author: Beatrix Boldt


