The research project getLIGHT (Novel light-inducible genome editing technology for crops) aimed to develop nothing less than a completely new genome editing method. In plant breeding, for example, this would enable the rapid and precise development of improved varieties without incurring expensive licensing fees for established methods such as CRISPR-Cas. Although the project period from June 2018 to May 2020 was not long enough to prove feasibility, the researchers from the Fraunhofer Institute for Molecular Biology and Applied Ecology IME were able to take important first steps towards their ambitious goal.
Bypassing licensing costs for CRISPR-Cas
"For research, CRISPR-Cas is great because you can quickly obtain results and publish them," explains project leader Stefan Schillberg. "But at the Fraunhofer Institute, we are interested in applications, and because others have the patents in CRISPR, our industrial partners would always have to pay for it." As a result, the costs in plant breeding would quickly become so high that no one would want to cover them. "My dream was to come up with my own process instead," the biotechnologist reveals. So-called quantum clusters were to form the approach to this. The necessary funding of around 500,000 euros came from the German Federal Ministry of Education and Research (BMBF).
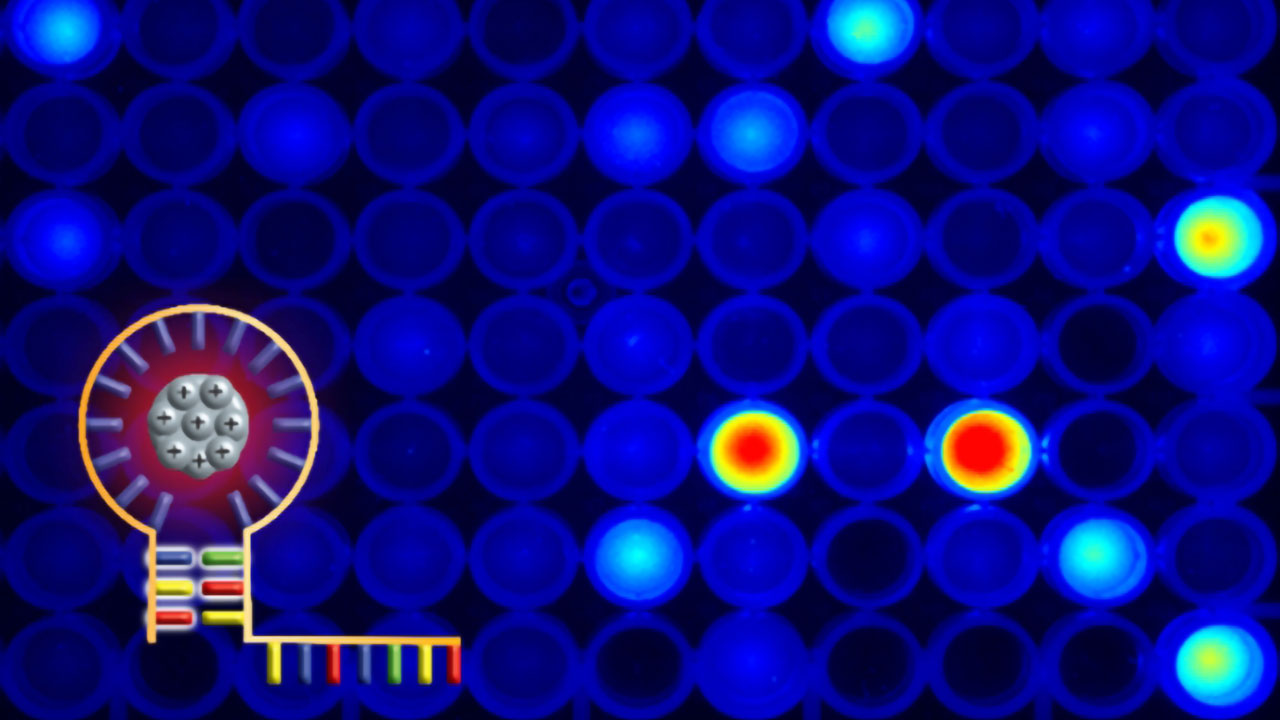
The researchers call a quantum cluster a connection between a short DNA strand, which can couple to the target sequence in the cell, and another DNA structure, for example a so-called loop, which can bind metal ions such as charged silver particles. When such a quantum cluster is excited with light, it fluoresces - with a different wavelength depending on the wavelength that falls on it. So far, such constructs have been used in detection methods, where the fluorescence provides information about the location of the quantum cluster.
In theory, quantum effect creates DNA breaks
"We wanted to use this effect to create strand breaks in DNA," Schillberg explains. It is known from theory that the photons emitted by fluorescence can set electrons in motion through a quantum physical effect known as the tunnel effect, thereby causing breaks in DNA strands. In this way, shorter or longer DNA segments could be excised and the affected gene inactivated. "The idea is that very precise interventions would be possible if you select the right sequence, because the energy transfer only takes place during excitation," says the researcher, highlighting an important advantage. CRISPR-Cas is also characterized by high precision, but because the molecular tools are permanently located in the cell in the process, Schillberg assumes that a time-limited light-induced process like getLIGHT would have fewer off-target effects, meaning that it would be less likely to alter DNA in places where it is not desired.
"With this project, we have achieved that we can produce relatively stable quantum clusters in a fairly simple way," Schillberg reports. The former would be an advantage to compete with other methods of genome editing. The latter is important if these nanopolymers are later to fulfill their function not only in the test tube, but in the cell. "These quantum clusters can be excited with light and also bind to their target sequence in vitro." There is also initial evidence that strand breaks are taking place there. "But we don't yet have clear proof that it works exactly as we imagine," says the project manager. The planned development was too fundamental for this and the project duration was too short. Since the end of the project, the research team has continued to work on its approach on the side and is looking for follow-up funding. "It would be a shame if we couldn't continue this," says Schillberg.
Open questions after the project ends
While the production of the quantum clusters proved to be unexpectedly simple, everything else is more complex than expected, summarizes the biotechnologist. If the method is to be developed further, it is primarily a matter of producing and testing further quantum clusters, and also of producing the right conditions, first in vitro and later in vivo, so that the strand break works as desired. "Whether we need to use a finer adjustment of the light irradiation for electron excitation or combinations of multiple quantum clusters and light sources - we have not yet been able to fully decipher that in the course of the project, for example," Schillberg says. He hopes that the means will be found to also resolve these questions - because he had not yet heard anyone in the research community say that getLIGHT approach could not work.
Author: Björn Lohmann


