3D structure of a fertilizer producing enzyme
Researchers of the University of Freiburg have deciphered the 3D structure of an enzyme that might be used for the biotechnological production of fertilizers and fuels.
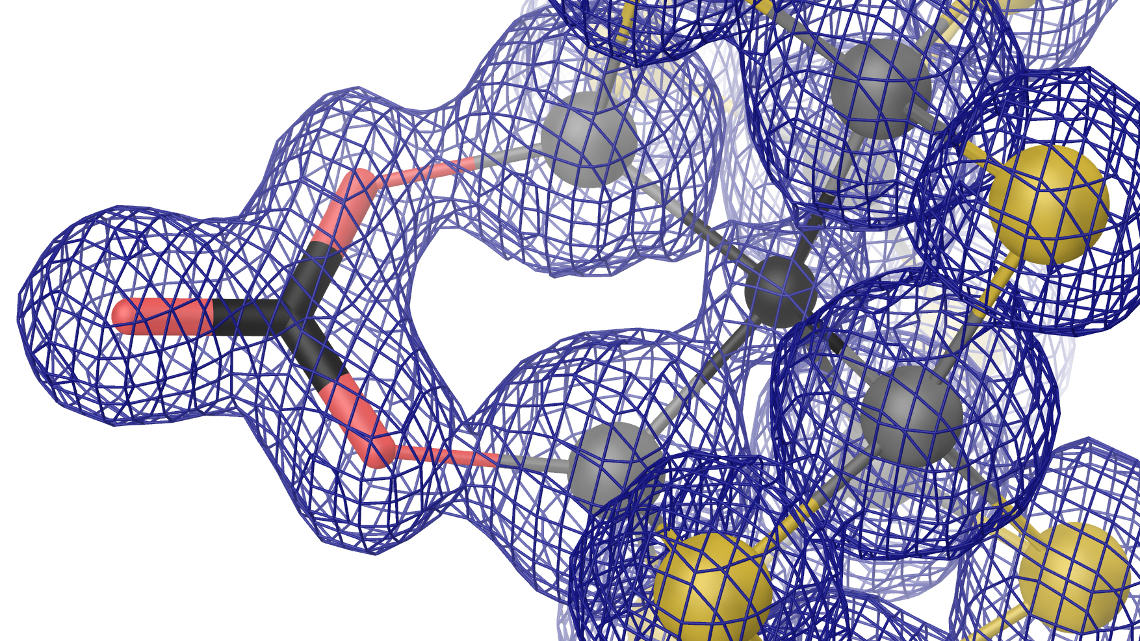
The vanadium-dependent nitrogenase is an enzyme that catalyses two important processes: On the one hand it converts atmospheric nitrogen (N2) to ammonia, on the other hand it reduces carbon monoxide (CO) to hydrocarbons. Today, both reactions are run on a big scale by chemical catalyses to produce ammonia and fuels for industry. In additon, ammonia is used as synthetic fertilizer to ensure the food production for at least half of the world’s population. Now, researchers of the University of Freiburg have described for the first time the 3D structure of the enzyme, which conducts both reactions in a biocatalytic way. They published their results in the scientific journal “Nature Chemical Biology”. The research was funded by the European Research Council (ERC) and by the German Research Foundation (DFG).
Special nitrogenase binds carbon monoxide
The vanadium-dependent nitrogenase derives from the soil bacterium Azotobacter vinelandii and belongs to the family of nitrogen-binding enzymes. For the biocatalysis the enzymes usually need an iron molybdenum cofactor. The special nitrogenase from Azotobacter vinelandii uses the molecule vanadium instead of molybdenum. Therefore, it is called vanadium(V)-dependent nitrogenase. This enzyme is also able to convert carbon monoxide to hydrocarbons. Due to its double function it is called “two-hit wonder”. However, the function of this complex and metal-containing enzyme system has only been partially explained so far.
3D structure of V-nitrogenase decoded
Researchers at the University of Freiburg have now decoded the spatial structure of the V-nitrogenase. “To do so, we have first crystallised the enzyme. Taking this as our basis, we used x-ray diffraction experiments to elucidate the spatial structure at the level of atomic resolution”, says Daniel Sippel of the biochemistry working group. “We also found that a bridging sulfide ion within the cofactor of V-nitrogenase is replaced with a chemically very different carbonate anion”, explains Oliver Einsle, professor for Biochemistry at the University of Freiburg. “What initially appeared to be only a minor difference has far reaching consequences on the geometric and electronic structure of the cofactor.”
Biotechnological potential
Nowadays, industrial processes are used to bind nitrogen and produce hydrocarbons. The processes were developed at the beginning of the 20th century. The Haber-Bosch process can generate synthetic ammonia fertilizer, and coal can be converted to hydrocarbons by the Fischer-Tropsch synthesis. The Haber-Bosch process requires high pressure and 400 to 500 degree Celsius, thus, a lot of energy is needed. Since the 1990s there is an increasing interest in the Fischer-Tropsch process, because fuel can be produced not only from coal, but also from bio-waste, wood, industrial waste gases, and agricultural residues. Fuel derived via the Fischer-Tropsch synthesis has been allowed for use in aviation since 2009. “Our long-term goal is to make nitrogenase biotechnologically useful”, says Einsle. The V-nitrogenase might be an alternative to the industrial chemical process in the future.
bp


